Which Alcohol Has the Strongest Intermolecular Forces
1-butanol has the strongest intermolecular force as the molecules are involved in strong hydrogen bonding. What type of intermolecular force causes the dissolution of CH3CH2OH in water.
Solved Which Of The Alcohols Studied Has The Strongest Chegg Com
As the carbon chain gets longer the contribution of the London dispersion forces becomes significant.
. Which of the alcohols studied has the strongest intermolecular forces of attraction. Explain using the results of this experiment. Name_ Score_50 Lab Day_ Post-lab.
Intermolecular Forces Introduction The purpose of. Water and ethyl alcohol will both have dipole-dipole interactions. This is because due to stronger intermolecular forces of attraction the evaporation of 1-butanol from the probe wa.
The weaker intermolecular forces. 1-butanol 1-butanol has the strongest intermolecular force as the molecules are involved in strong hydrogen bonding. 1-Butanol has the strongest intermolecular forces of attraction while methanol has the weakest intermolecular forces of attraction.
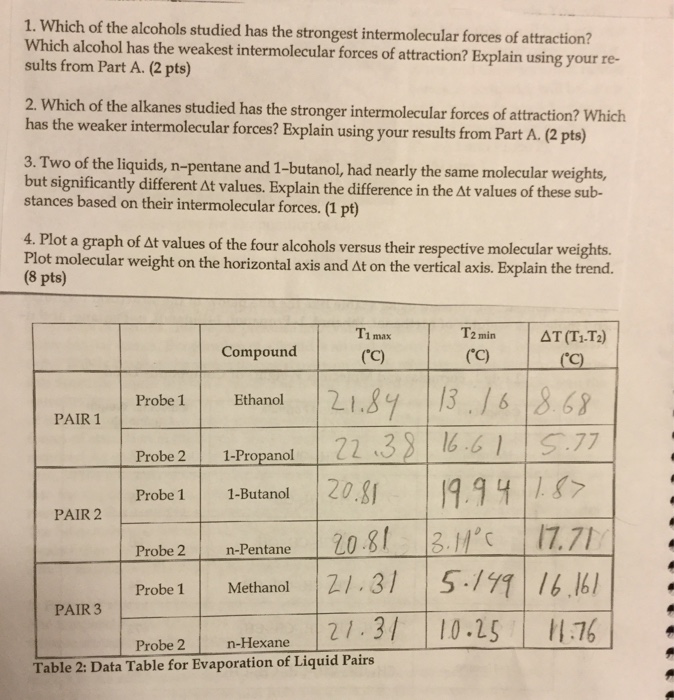
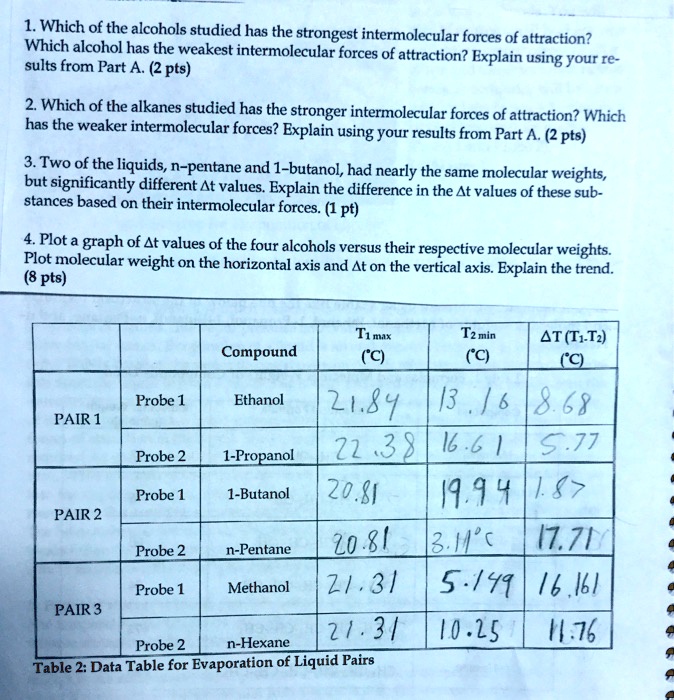
View the full answer. Intramolecular forces can be regarded as the forces that hold atoms together this force serve as binding that let the atoms stays together within a molecule. Plot a graph of Δt values of the four alcohols versus their.
Forces is as follows. As a result butanol will have strongest intermolecular force of attraction because it contains more number of carbon atoms and also it has more electronegative oxygen atom. View Intermolecular Forces Post-Labdocx from CHM 111 at Arizona State University.
For example ethanol with a molecular weight MW of 46 has a boiling point of 78. The weakest intermolecular forces 2. Water 1-propanol ethanol acetone hexane and.
How do you identify intermolecular forces. Hydrogen bonding is the strongest type of intermolecular force. Expert Answer 100 13 ratings 1.
Whereas n-pentane will have the weakest intermolecular force of attraction between its atoms. Short chain alcohols have intermolecular forces that are dominated by H-bonds and dipoledipole so they dissolve in water readily infinitely for methanol and ethanol. Sep 7 2017 The strongest intermolecular forces in methanol are hydrogen bonds.
The substance that can be regarded as the one with stronger intermolecular forces in this question is Water. Which alcohol has strongest intermolecular forces. Up to 24 cash back Which alcohol has the strongest intermolecular force of attraction.
I think the cutoff is about 5 carbons - when you have n-pentanol this molecule is sparingly. In order of magnitude intermolecular forces include i hydrogen bonding where hydrogen is bound to a strongly. Ethanol has an OH group O bonded to H which means that it can form hydrogen bonds between molecules.
Which of the alkanes studied has the stronger intermolecular forces of attraction. Technically they will both have Hydrogen bonding which is a type of dipole-dipole. CH3CH2OH has the strongest intermolecular forces because it has the strongest dipoledipole forces due to hydrogen bonding.
What is the strongest intermolecular force to be over ethanol.

Solved 1 Which Of The Alcohols Studied Has The Strongest Intermolecular Forces Of Attraction Which Alcohol Has The Weakest Intermolecular Forces Of Attraction From Explain Sults Using Part A 2 Pts Your Rc

Solved 2 Which One Of The Three Alcohols Had The Strongest Chegg Com

3 Trends That Affect Boiling Points Master Organic Chemistry Organic Chemistry Chemistry High School Chemistry
Comments
Post a Comment